Figure 1: Workflow for BCMA lead molecules project. From 4ml of rabbit whole blood, we identified 5 lead mAbs molecules for human BCMA targets with verified functional data and antibody sequences. We also created a B cell seed library for the human BCMA target which presumably contains 10 thousands Flow positive BCMA binders providing a good resource for additional screening.
先导抗体申请表
研究案例:BCMA-先导抗体分子开发及CAR-T应用
1、简介:
多发性骨髓瘤(MM,Multiple myeloma):仅次于非霍奇金淋巴瘤的第二大常见血液学恶性肿瘤,几乎所有患者预后仍会复发,被认为是无法治愈的疾病,新治疗方案开发需求迫切;
BCMA是治疗MM理想的抗原靶点:除了成熟B淋巴细胞及浆细胞表面,BCMA在其他组织细胞中几乎不表达,但它在所有MM细胞中高表达;
市场开发赛道火热:葛兰素史克的Blenrep(2020上市);百时美施贵宝与蓝鸟生物的Ide-cel(FDA优先审评);南京传奇和杨森的LCAR-B38M(三期临床)……
2、BCMA开发流程:
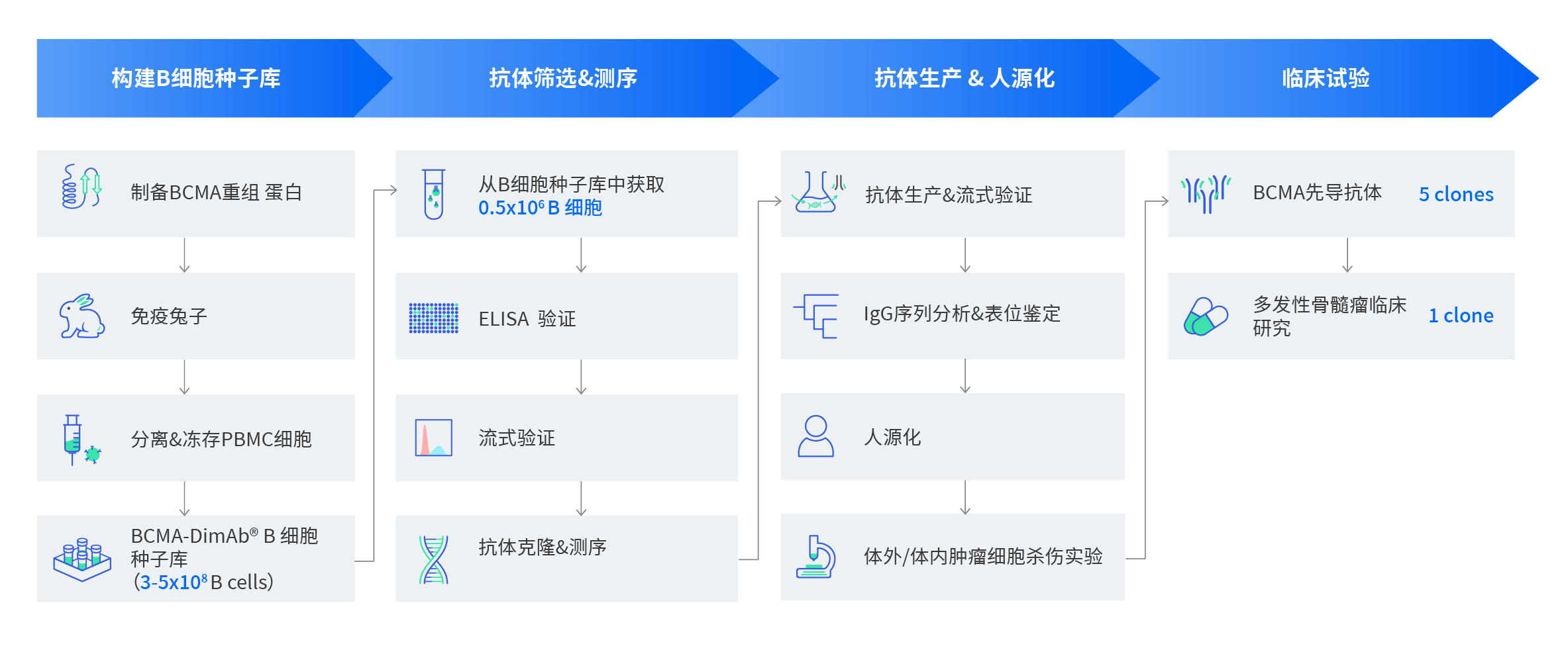
首先,我们制备了大量的BCMA重组蛋白作为抗原免疫兔子,所有蛋白经SDS-PAGE和多种结合实验验证具有高纯度及高活性。我们从兔血清中分离出3~5 X108 B细胞,并快速冻存至液氮中,作为BCMA-B细胞种子库长期保存。从4mL兔全血中,我们筛选出70个ELISA阳性的B细胞克隆,其中,13个克隆呈现流式阳性。我们对这13个克隆进行单抗克隆和测序,通过系统发育分析和表位比较,鉴定出5个克隆。将这5个克隆人源化后应用于CAR-T研究,它们都显示出与蓝鸟公司的抗BCMA huC11d5.3克隆具有相似的肿瘤细胞杀伤效果。
2.1、BCMA功能性蛋白抗原开发
为了确保BCMA重组表达蛋白具有功能活性,我们对BCMA蛋白抗原进行了BCMA受体-配体互作验证,同时也验证了BCMA重组蛋白和huC11D5.3中和性抗体的体外结合能力(来源于蓝鸟公司在临床三期的CAR-T结构bb2121)。
Functional BCMA protein used as immunogen
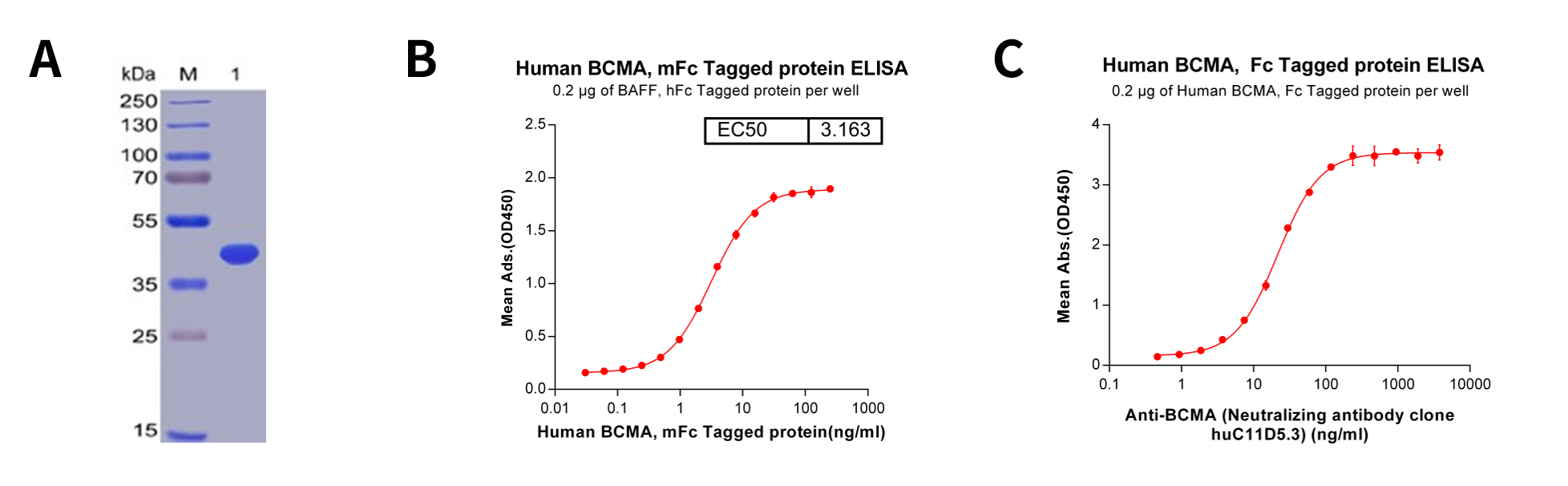
Figure 2: Quality analysis of purified human BCMA protein, Fc-tagged. A. Human BCMA, Fc-tagged on SDS-PAGE under reducing condition. B. ELISA plate pre-coated by 2 μg/ml (100 μl/well) Human BAFF, hFc tagged protein can bind Human BCMA, Fc- tagged protein in a linear range of 0.03-15.625 ng/ml. C. ELISA plate pre-coated by 2 μg/ml (100 μl/well) Human BCMA, Fc-tagged protein can bind Anti-BCMA (huC11D5.3) (Its variable region was used to construct scFv portion of CAR-T Idecabtagene vicleucel (bb2121). ) in a linear range of 3.71-22.29 ng/ml.
2.2、BCMA先导分子发现和B细胞种子库
利用重组单克隆抗体平台,我们从4ml免疫后兔全血中筛选获得了70株靶点结合阳性B细胞克隆,其中13株克隆进行了功能验证。而这些只是BCMA B细胞种子库中的一小部分,后期可以随时扩大筛选。
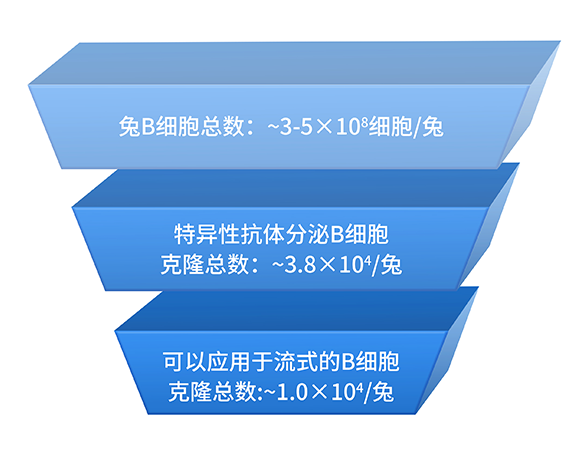
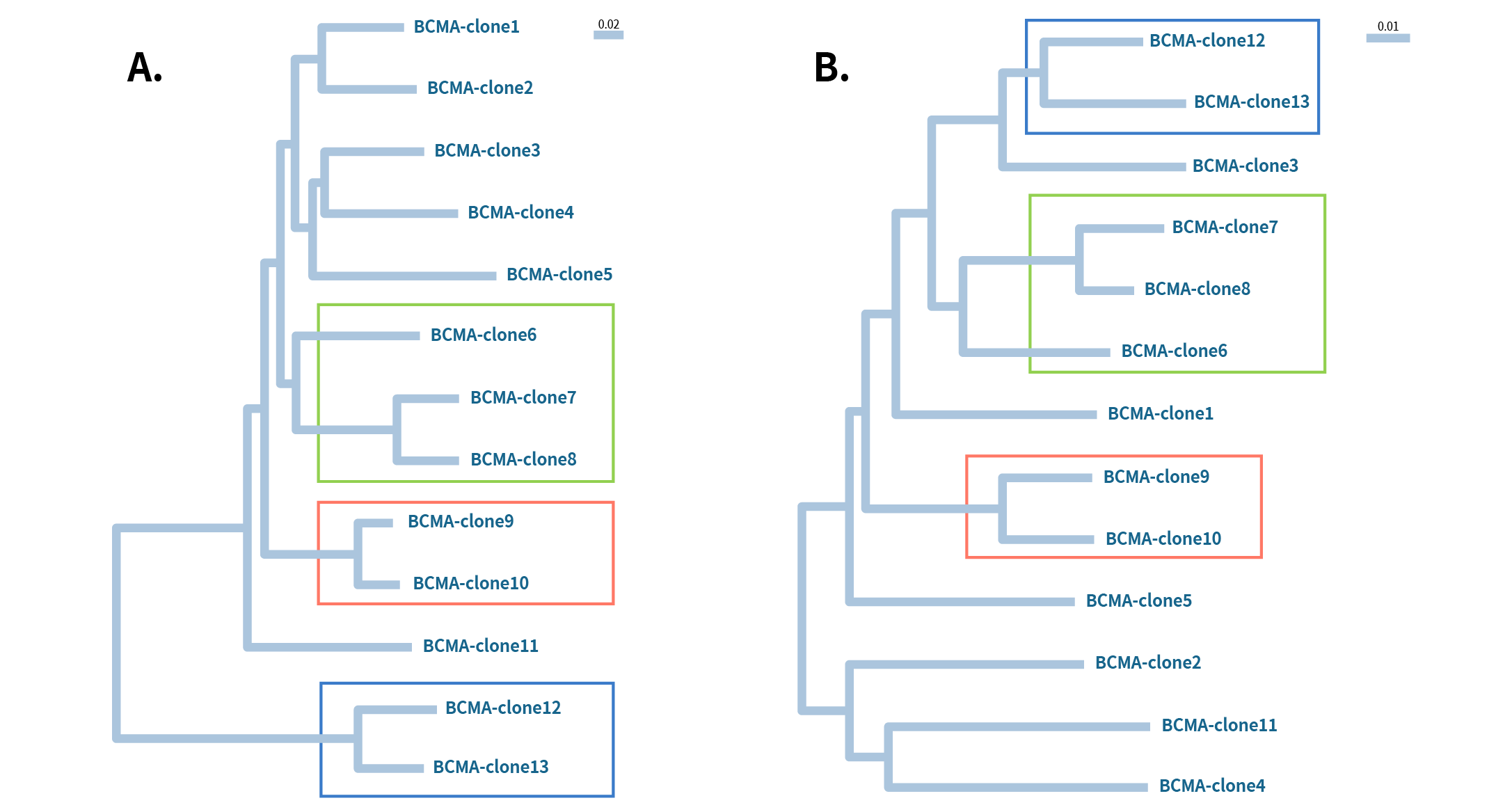
Figure 3: Phylogenetic analysis of 13 different Anti-BCMA clones A) heavy chain and B) Light chain. All these clones work for flow application. The boxed regions indicate heavy and light chains of the same clone come from the same lineage group.
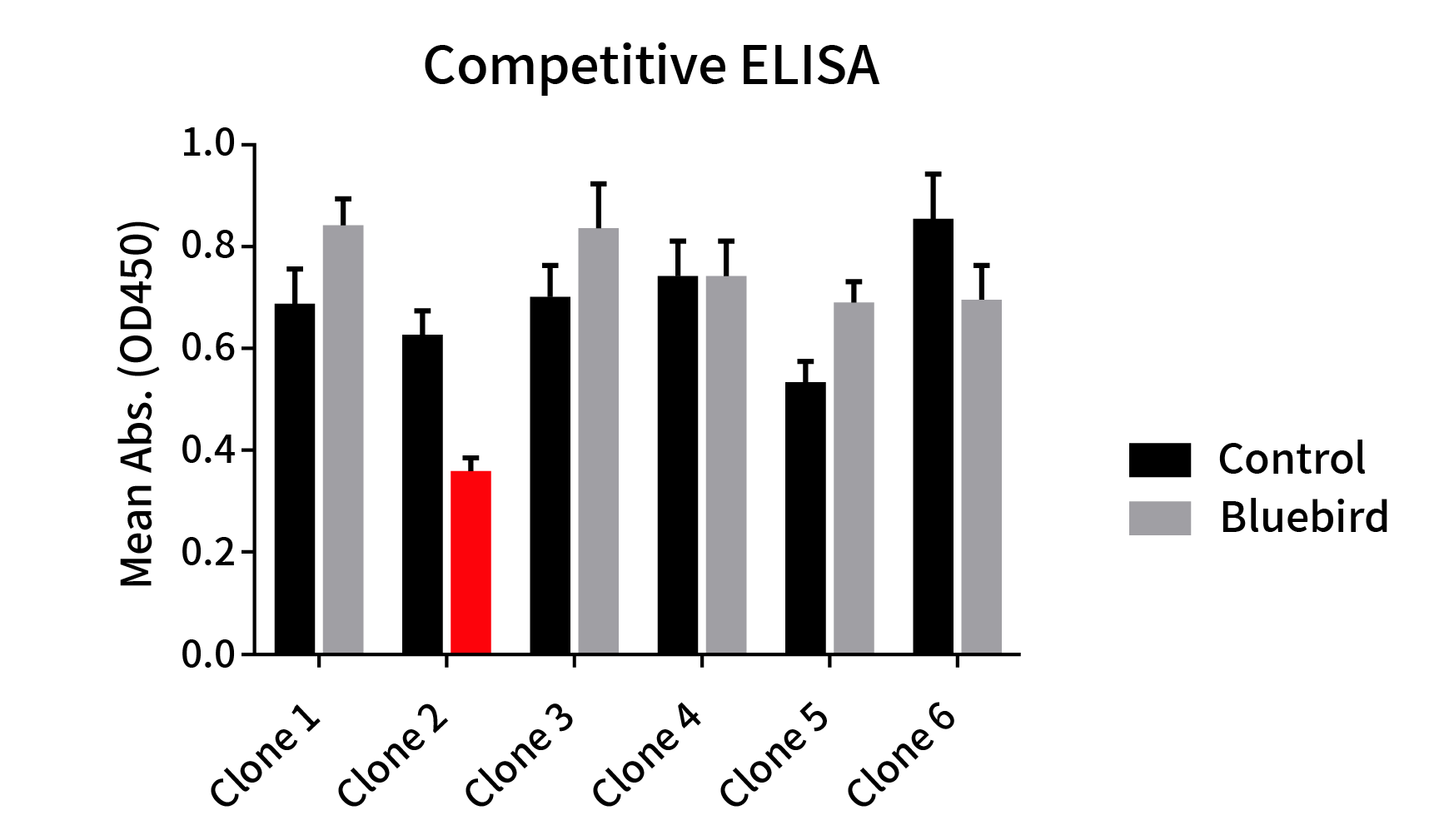
Figure 4: Epitope comparison between different anti-BCMA clones and anti-BCMA huC11D5.3 clone (Bluebird bb2121). ELISA plate was coated with recombinant BCMA-hFc fusion protein, followed by pre-blocking with huC11D5.3 antibody (Grey bar) or rabbit control IgG (Black bar) and then different rabbit DimAbs antibodies were added to check the competitive inhibition of huC11D5.3. One clone exhibits the strongest inhibition (Red bar). This data indicated that one clone binds to the same epitope as bb2121.
2.3、BCMA抗体工程改造
根据重组单克隆抗体平台提供的BCMA抗体基因序列信息,可以让我们直接对筛选验证的5株高亲和力克隆株进行抗体人源化工程改造,同时构建了双特异性BiTE分子和CAR分子,并进行体外功能验证。(目前已有克隆株商业转化)
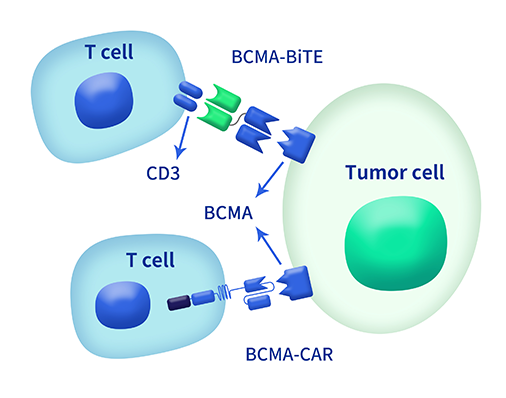
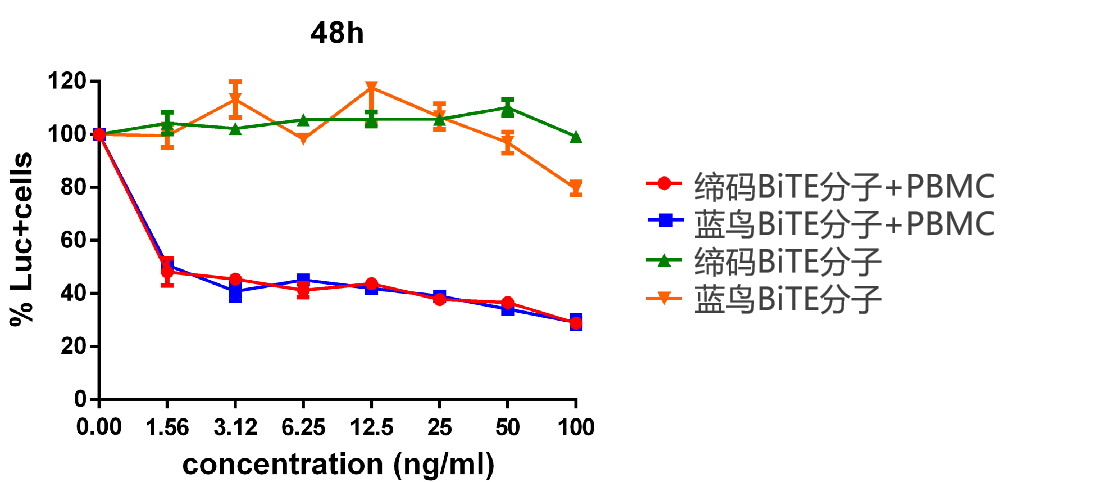
BCMA双特异性BiTE分子体外功能分析
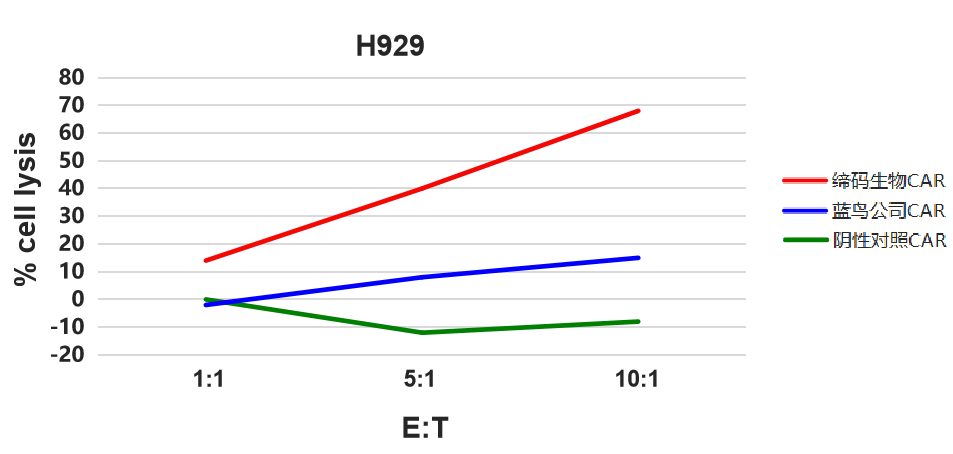
Figure 5. 人源化BCMA-BiTE分子(DM6克隆)瘤细胞杀伤检测
BCMA CAR 分子体外功能分析
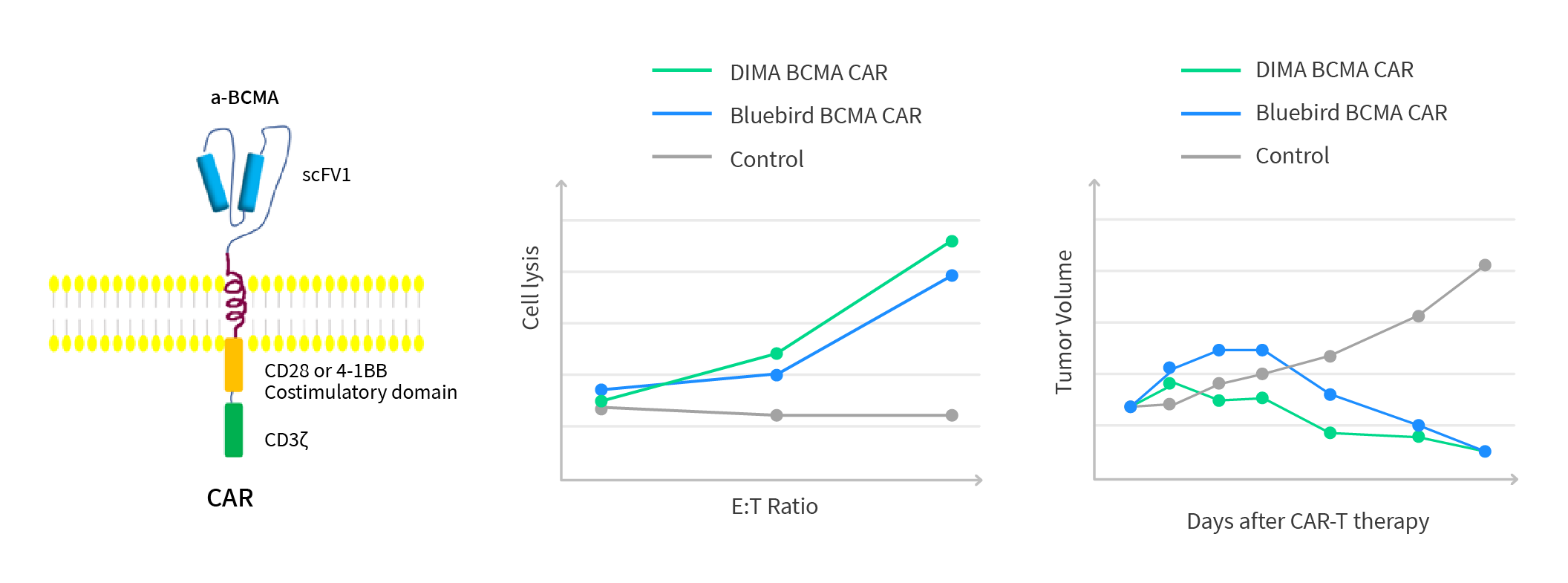
Figure 6: In-vivo testing of humanized anti-BCMA lead mAbs molecules. The preliminary tumor cell killing efficacy testing data is proprietary. It indicated that our 5 humanized CARs are comparable or better than BMK.
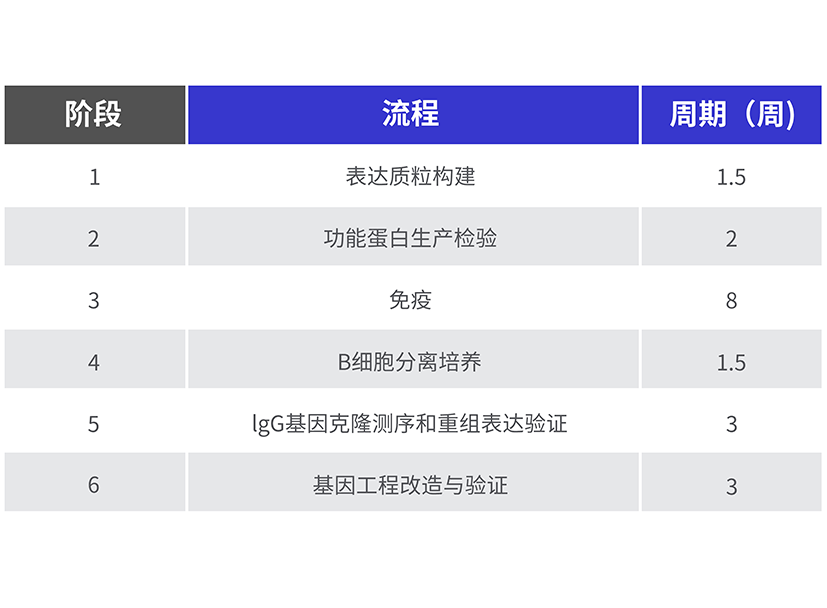
3、BCMA 开发周期:
项目总开发周期:约4.5个月