First full length rabbit sourced mAb approved by FDA
Time:2020-03-14
Time:2020-03-14
FDA approved the listing of Eptinezumab, a CGRP antibody of Danish Lundbeck pharmaceutical, under the trade name of Vyepti, to prevent migraine On February 22, 2022. Since 1986, FDA has approved more than 90 new monoclonal antibody drugs, most of which are from mice, followed by phage display library. In October 2019, the first rabbit monoclonal antibody Beovu (scFv antibody fragment) was approved by FDA to treat wet AMD. Eptinezumab is the first approved full-length rabbit monoclonal antibody drug.
Comparing with other animals, the rabbit has a unique B cell development process and is more distant from human than rodent. Besides these, rabbit B cells utilize a dual affinity maturation mechanism, including gene conversion and SHM (somatic hyper-mutation). At the same time, the rabbit IgG has a unique protein structure different from the mouse IgG, including one subtype of IgG, more noncanonical C-C for light chain, great variation in length and sequence for CDR3, etc. These differences might make rabbit produce antibodies with high affinity and wide diversity. In recent years, rabbit monoclonal antibodies are getting popular in different immunological assay development, especially for immunohistochemistry application. So far, 5 FDA approved companion diagnostic IHC antibodies are rabbit monoclonal antibodies.
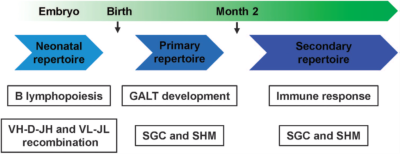
Figure 1. Development of rabbit B cell and antibody spectrum
Compared with mouse McAb, rabbit McAb has following advantages:
(1) Higher antibody diversity;
(2) Small molecule can produce strong immune response;
(3) Higher antibody affinity;
(4) More quantity of B lymphocyte is beneficial to downstream research;
(5) The antibody gene is simple and IgG has only one subtype, which are convenient for high-throughput molecular biological operation;
(6) The humanization of rabbit McAb is simpler, and it is easy to achieve more than 95% of the humanization ratio.

Figure 2. Chimeric rabbit / human Fab and humanized rabbit Fab
Compared with the traditional monoclonal antibody preparation platform for hybridoma, we do not need to carry out hybridoma fusion. We can directly obtain positive B cells from peripheral blood of immunized animals after animal immunization and sequence the gene of monoclonal antibody. More than 1000 positive B cell clones can be obtained from a single immunized animal, and the overall positive clone rate is far higher than that of the traditional hybridoma platform. From a large number of clones obtained from DimAb™ development platform, we can more efficiently screen out recombinant monoclonal antibodies with high specificity, high sensitivity and high affinity. We can also directly edit, optimize and identify the downstream antibody of the recombinant monoclonal antibody (such as humanization of antibody, affinity maturation, construction of cell lines, etc.). At present, DimAb™ platform has achieved a high-throughput project development of rabbit and mouse recombinant monoclonal antibodies. We are focusing on immune oncology targets and providing high-quality antibody services for global research, diagnosis and treatment.
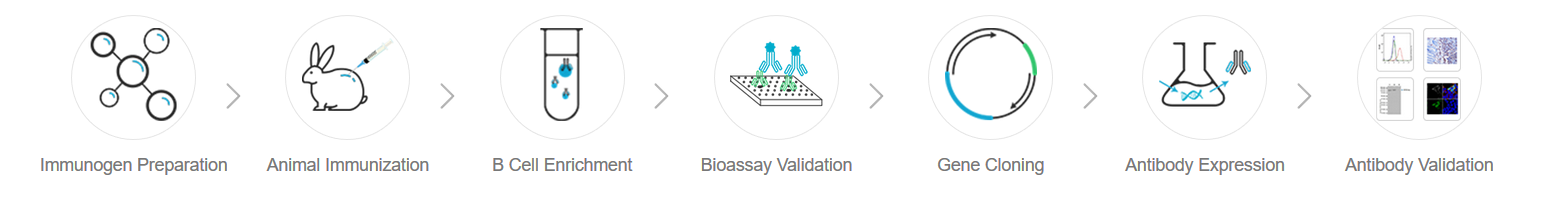
Figure 3. DimAb™ recombinant monoclonal antibody platform process